Physical Address
Let’s first look at activation energy. Which of the following requires us to put in more energy: walking or driving 10 miles? If you said walking, you are correct. (In a car, all we have to do is press the gas pedal!) An enzyme—the car in our example—reduces the amount of input energy necessary to undertake a chemical reaction. By lowering the input energy, or activation energy, of a reaction, the reaction can occur much more quickly. This brings us to reaction kinetics.
Enzymes
- Kinetics
- General (catalysis)
- Enzyme “catalyzes” a reaction, meaning it changes the kinetics of a reaction without being used up
- Kinetics (how fast a reaction occurs) vs Thermodynamics (whether and what direction a reaction will occur)
- Enzyme makes a reaction go faster by lowering the activation energy
- Enzymes lower the activation energy by stablizing the transition state (high energy state between reactant and product)
- Michaelis-Menten = reaction rate (V) vs substrate concentration [S] plot
- Vmax = maximum reaction rate = V where [S] is infinity
- Km = Michaelis constant = [S] where V is half of Vmax
- Cooperativity
- Positive cooperativity = binding of substrate from one subunit makes another subunit more likely to bind
- Negative cooperativity = binding of substrate from one subunit makes another subunit less likely to bind
- General (catalysis)
- Feedback regulation
- The product of a pathway inhibits the pathway.
- For example, hexokinase, the first enzyme in glycolysis, is inhibited by its product glucose-6-phosphate.
- Inhibition – types
- Competitive inhibition
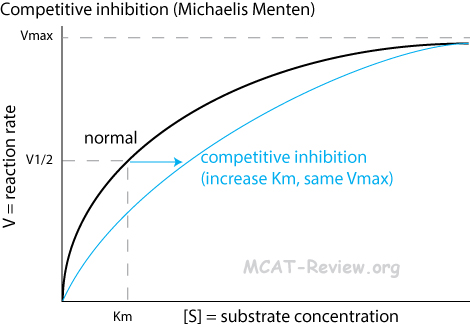
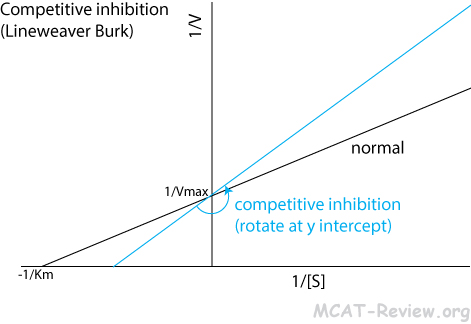
- An inhibitor competes with the substrate for binding to the active site.
- Competitive inhibition increases Km (the amount of substrate needed to achieve maximum rate of catalysis).
- Competitive inhibition does NOT change Vmax (the maximum possible rate of the enzyme’s catalysis).
- Michaelis Menten plot: less hyperbolic curve
- Lineweaver Burk plot: counterclockwise rotation around the y intercept
- You can overcome competitive inhibition by providing more substrate.
- Non-competitive inhibition
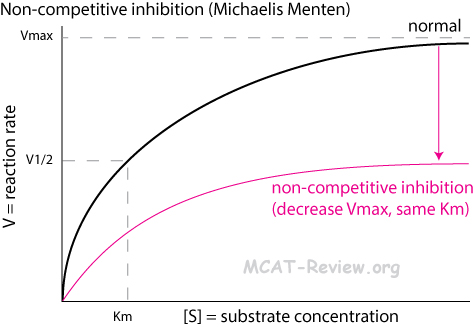
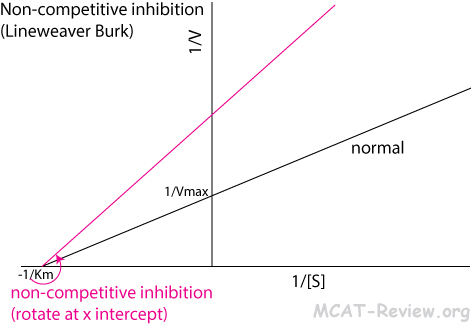
- An inhibitor binds to an allosteric site on the enzyme to deactivate it.
- The substrate still have access the active site, but the enzyme is no longer able to catalyze the reaction as long as the inhibitor remains bound.
- Non-competitive inhibition decreases Vmax (the maximum possible rate of the enzyme’s catalysis).
- Non-competitive inhibition does NOT change Km (the amount of substrate needed to achieve the maximum rate of catalysis).
- Michaelis Menten plot: lower plateau
- Lineweaver Burk: counterclockwise rotation around the x intercept
- You can’t overcome non-competitive inhibition by adding more substrate.
- Mixed
- Michaelis Menten plot: lower plateau AND less hyperbolic curve
- Lineweaver Burk plot: both y and x intercept changes (y intercept shifts up, x intercept shifts right)
- Uncompetitive inhibition
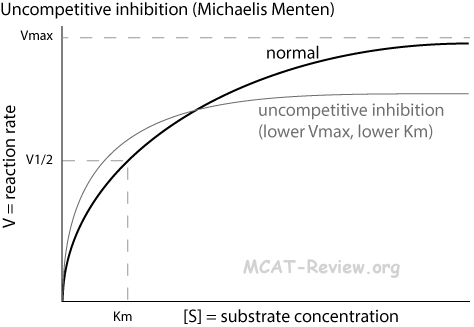
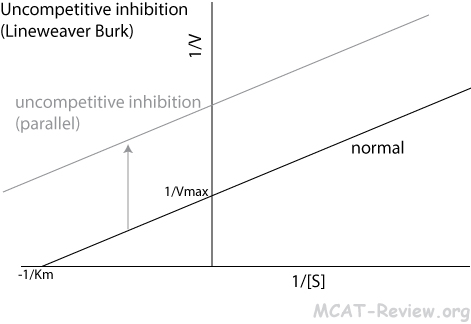
- Inhibitor binds the enzyme-substrate complex
- Michaelis Menten plot: lower plateau but more hyperbolic curve
- Lineweaver Burk plot: parallel shift upward
- Competitive inhibition
- Regulatory enzymes
- Allosteric enzymes: binding at allosteric site -> change in enzyme conformation -> change in active site to bind substrate more easily
- Covalently-modified enzymes: phosphorylation of an enzyme makes it active/inactive
- Zymogen: inactive until activated by another enzyme. Eg: Pepsinogen (inactive) -> pepsin (active); trypsinogen (inactive) -> trypsin (active)
MCAT Review and MCAT Prep Online – mcat-review.org – Copyright @ 2008-2018 – All Rights Reserved | privacy policy | USMLE Review | Physics solver
Enzymes for the MCAT: Everything You Need to Know
Learn everything you need to know about enzymes—one of the most heavily tested science topics on the MCAT

(Note: This guide is part of our MCAT Biochemistry series.)
Part 1: Introduction to enzymes
Part 2: General enzyme characteristics
a) Catalytic activity
b) Enzyme-substrate binding
c) Types of enzymes
d) Cofactors, coenzymes, and vitamins
e) Catalytic amino acids
Part 3: Enzyme kinetics and inhibition
a) Michaelis-Menten and Lineweaver-Burk
b) Competitive inhibition
c) Uncompetitive inhibition
d) Mixed and noncompetitive inhibition
Part 4: Regulating enzyme activity
a) Local environment conditions
b) Covalent modification
c) Allosteric regulation
d) Zymogens
e) Cooperativity
f) Feedback regulation
Part 5: High-yield terms
Part 6: Enzymes practice passage
Part 7: Enzymes practice questions and answers
Part 1: Introduction to enzymes
Enzymes are one of the most heavily tested science topics on the MCAT, and they are an important part of our everyday life. Each cell in our body carries out many of its functions using enzymes, and misregulation of these enzymes is responsible for a wide scope of human diseases, such as cancer and hypertension.
Many students struggle with enzymes on the MCAT, often losing valuable points on questions testing topics from enzymatic inhibition to feedback regulation. In this guide, we will break down the content you need to know—no more and no less—to study enzymes for the MCAT. All of the terms bolded throughout the guide will be defined in Part 5 of the guide, but we encourage you to create your own definitions and examples as you move through this resource so that they make the most sense to you!
In addition to knowing the content, you will also need to know how to analyze the various graphs, equations, and terms related to enzymes that the MCAT presents. At the end of this guide, there is an MCAT-style enzyme practice passage and standalone questions that will both test your knowledge and show you how the AAMC likes to ask questions.
Let’s get started!
Part 2: General enzyme characteristics
a) Catalytic activity
Enzymes are biological catalysts, and a catalyst is defined as a substance that speeds up the rate of a chemical reaction without being consumed itself. For example, let’s say you need to get from point A to point B, and they are 10 miles apart. You could walk, which would likely take a while. Or you could drive a car and arrive a lot faster. In this case, the car serves as our catalyst. Note, the distance is not changing, but the speed with which you get there is faster.

In the same way that a car speeds up our transportation, an enzyme serves to speed up the rate of a biochemical reaction. As a result, an enzyme is classified as a biological catalyst, which has the following properties (we will dive into each one):
- Lowers activation energy of a reaction
- Affects the kinetics of the reaction, but not the thermodynamics (∆G) or equilibrium constant
- Regenerates itself
Let’s first look at activation energy. Which of the following requires us to put in more energy: walking or driving 10 miles? If you said walking, you are correct. (In a car, all we have to do is press the gas pedal!) An enzyme—the car in our example—reduces the amount of input energy necessary to undertake a chemical reaction. By lowering the input energy, or activation energy, of a reaction, the reaction can occur much more quickly. This brings us to reaction kinetics.
An enzyme affects the kinetics of a reaction by speeding up the rate of the reaction. Note, the reaction rate is the rate at which reactants are consumed or the rate at which products are made. Importantly, enzymes DO NOT affect the thermodynamics (∆G) or equilibrium constant of a reaction.
Let’s look at another example to illustrate this point. We have a machine that converts red spheres into red cubes. Normally, if you give the machine 10 red spheres, it will produce 5 red cubes in 1 hour. Now, let’s say a catalyst acts on the machine—if you give the machine 10 red spheres, it will produce 5 red cubes in 1 minute.
The kinetics of the machine are affected as it can now work faster, but the thermodynamics and equilibrium constant stay the same. In each case, 10 red spheres are made into 5 red cubes, regardless of how fast the machine can do it.
We’ve illustrated these effects below in a commonly drawn reaction diagram:

Finally, enzymes are regenerated during their catalytic cycles. This is very important as regeneration reduces the amount of protein that a cell has to make in order to carry out biochemical reactions. For example, cells use a lot of ATP, and most of this ATP is generated by ATP synthase, an enzyme. (As a brief aside, enzymes often end with an -ase!) If each ATP synthase could only synthesize one ATP molecule, the cell would be overrun by the ATP synthase enzymes. The ability of each ATP synthase—and other enzymes—to reset itself after each cycle is absolutely essential to our survival.
In the figure below, we show you how regeneration would make a great difference in the number of ATP synthases needed for a cell if it could not regenerate itself for multiple catalytic cycles.

b) Enzyme-substrate binding
We’ve talked about the general properties of enzymes, and now we will dip our toes into more of the nitty-gritty details. We often talk about chemical reactions by writing out reaction schemes such as A → B → C. For enzymes, we can draw out a reaction scheme that can help us better understand what exactly is going on, and it looks something like the following:
We’ll come back to the overall reaction scheme when we look at enzyme inhibition in the next section, but let’s zoom into the ES, or enzyme-substrate complex.
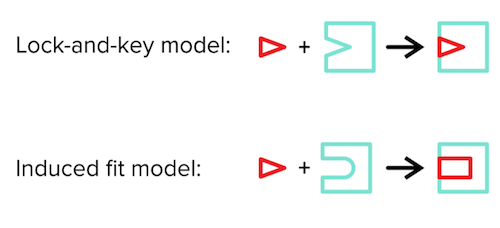
There are two models for describing the enzyme-substrate complex: 1) the lock-and-key model and 2) the induced fit model. The lock-and-key model describes the substrate as the “key” and the enzyme as the “lock.” Without changing any conformations, the key should fit nicely into the lock for the two to bind.
The induced-fit model (which is generally the more accepted model) states that enzyme and substrate conformations do not have to be as rigid as suggested by the lock-and-key model. Instead, upon substrate binding to the enzyme, both will undergo slight conformational changes to improve their binding to one another.
c) Types of enzymes
There are six well-known types of enzymes that the MCAT wants you to know:
| Enzyme type | Function | Example |
|---|---|---|
| Isomerase | Catalyzes an isomerization reaction, which is an intramolecular rearrangement of bonds in a molecule | An enzyme that converts a cis double bond into a trans double bond |
| Ligase | Catalyzes the joining of two molecules | An enzyme that seals the gap between two adjacent Okazaki fragments |
| Transferase | Catalyzes the transfer of a functional group from one molecule to another molecule | A kinase that adds a phosphate group from ATP to a protein substrate |
| Lyase | Catalyzes the breaking of a molecule without the use of water | An enzyme that breaks a bond between two nucleotides without using water |
| Hydrolase | Catalyzes the breaking of a molecule by adding water | An enzyme that breaks a bond between two nucleotides by adding water |
| Oxidoreductase | Catalyzes the transfer of electrons between molecules | An enzyme that transfers extra electrons from an NADH electron carrier to a protein substrate |









