Physical Address
The islets of Langerhans contain three important types of cells: α-cells, β-cells, and δ-cells.
Carbohydrate Metabolism for the MCAT: Everything You Need to Know
Learn key MCAT concepts about carbohydrate metabolism, plus practice questions and answers
(Note: This guide is part of our MCAT Biochemistry series.)
Part 1: Introduction
Part 2: Digestion of carbohydrates
a) Enzymatic breakdown
b) Pancreatic regulation
c) Glycogenesis and glycogenolysis
Part 3: Glycolysis and fermentation
a) Glycolysis
b) Lactic acid fermentation
c) Gluconeogenesis
Part 4: Pyruvate oxidation and the TCA cycle
a) Mitochondrial structure
b) Pyruvate oxidation
c) The citric acid cycle
Part 5: Electron transport chain and oxidative phosphorylation
a) The electron transport chain
b) The electrochemical gradient
c) Oxidative phosphorylation
Part 6: Pentose phosphate pathway
Part 7: High-yield terms
Part 8: Passage-based questions and answers
Part 9: Standalone questions and answers
Part 1: Introduction
As modernization brings about technological advancements and higher standards of living, it seems to be a double-edged sword. The increased consumption of refined carbohydrates, or highly processed carbohydrates, has become a global problem. In addition to the United States, China and India—two countries that have been lauded for their economic gains these last few decades—are seeing unprecedented levels of obesity and cardiovascular diseases. Thus, it is more critical than ever that healthcare providers understand the biochemical pathways involved with metabolizing the foods we eat and their potential clinical implications.
In this guide, we’ll discuss the digestion and metabolism of one of the MCAT’s favorite macromolecules: carbohydrates. For more information on the carbohydrates and carbohydrates structures themselves, feel free to refer to our guide on carbohydrates.
As we discuss the various pathways involved, make sure to study around the following questions:
- When does this pathway occur? (e.g., times of starvation)
- Where does this pathway occur? (e.g., the mitochondrial matrix)
- Why does this pathway occur? (e.g., to generate energy)
- How does this pathway occur? (e.g., what is the mechanism?)
These four questions will help you stay focused on the big picture and not get too lost in the details. At the end of this guide, you will have the opportunity to apply your knowledge with a practice passage and questions.
Part 2: Digestion of carbohydrates
a) Enzymatic breakdown
Amylase is the key enzyme involved in the hydrolysis of large polymeric carbohydrates, such as starch, into smaller units. These large carbohydrates can be broken down into monosaccharides and disaccharides: sugar monomers and their linked products.
Amylase can be found in the saliva as salivary amylase and in the pancreatic secretions as pancreatic amylase. Salivary amylase takes action in the mouth, while pancreatic amylase is secreted into the small intestine.
Disaccharidases then further break down the disaccharides in the duodenum. Maltase, sucrase, and lactase break down maltose, sucrose, and lactose, respectively. Thus, we are left with three monomeric carbohydrates: glucose, galactose, and fructose.
b) Pancreatic regulation
In addition to secreting pancreatic amylase, the pancreas is also responsible for the secretion of three essential hormones. These hormones are manufactured by distinct pancreatic cells, which can be found grouped together in structures called islets of Langerhans.
The islets of Langerhans contain three important types of cells: α-cells, β-cells, and δ-cells.
- Glucagon is secreted by α-cells when blood glucose levels are low. This hormone promotes both anabolic and catabolic pathways that increase glucose concentration in the blood, such as glycogenolysis and gluconeogenesis.
- Insulin is released by β-cells when blood glucose levels are high. It promotes catabolic pathways, such as glycolysis, to derive energy from glucose. It also promotes anabolic pathways, such as glycogenesis, fatty acid synthesis, and peptide synthesis.
- Somatostatin is released by δ-cells in response to high blood glucose and amino acid levels. It inhibits the secretion of insulin and glucagon.
For information on additional important roles of the pancreas, refer to our guide on the endocrine system.
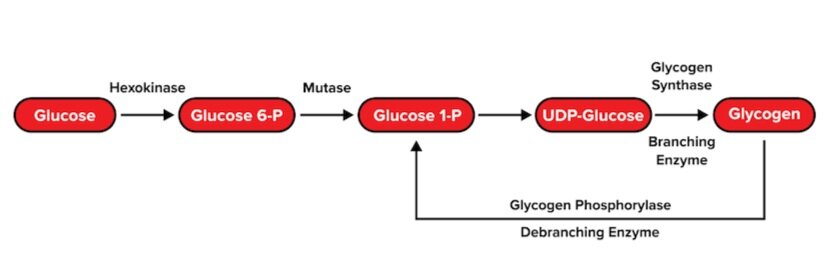
c) Glycogenesis and glycogenolysis
Recall that the body stores glucose monomers in the form of glycogen: a large, branching polysaccharide. To form glycogen, glucose monomers are polymerized via glycogenesis. This process occurs through multiple steps:
- Each glucose 6-phosphate monomer is converted into glucose 1-phosphate.
- A biomolecule known as uridine diphosphate (UDP) is attached to the glucose molecule.
- The glucose monomer is either added to a protein called glycogenin to initiate a glycogen chain or added to a growing glycogen chain by glycogen synthase. Uridine diphosphate is recycled. Glycogen synthase connects glucose monomers linearly using α-1,4 glycosidic linkages.
- At certain points, an enzyme known as branching enzyme hydrolyzes one of these linkages. This breaks off an oligosaccharide that the enzyme uses to start a new branch with an α-1,6 glycosidic linkage.
Glycogen is broken back into its glucose monomers via glycogenolysis. Similar to glycogenesis, there are two critical enzymes that will catalyze the reverse reactions of glycogenesis.
- An enzyme known as glycogen phosphorylase breaks the α-1,4 glycosidic linkages in a linear chain until it reaches the branching point.
- Debranching enzyme hydrolyzes the α-1,4 glycosidic linkage and relocates the resulting oligosaccharide to the end of another linear chain.
- Debranching enzyme then hydrolyzes an α-1,6 glycosidic linkage between the branched glucose molecule and the linear chain, resulting in the release of a single glucose monomer.
Part 3: Glycolysis and fermentation
a) Glycolysis
Glycolysis is the process by which a glucose molecule is converted into two molecules of pyruvate. It typically occurs in the cytoplasm. In addition to 2 pyruvate molecules, each glucose molecule that undergoes glycolysis will also result in the production of 2 NADH and 4 ATP molecules. However, during the process, 2 ATP molecules are consumed. Thus, the net products of glycolysis are 2 pyruvate molecules, 2 NADH, and 2 ATP. (These NADH molecules will be quite useful as electron carriers in the electron transport chain, which we will discuss later.)
The following diagram illustrates every step of glycolysis; however, only a handful of these are particularly high yield. While you won’t need to memorize each step of glycolysis and its related enzymes, it may be useful to be familiar with the function of each enzyme.
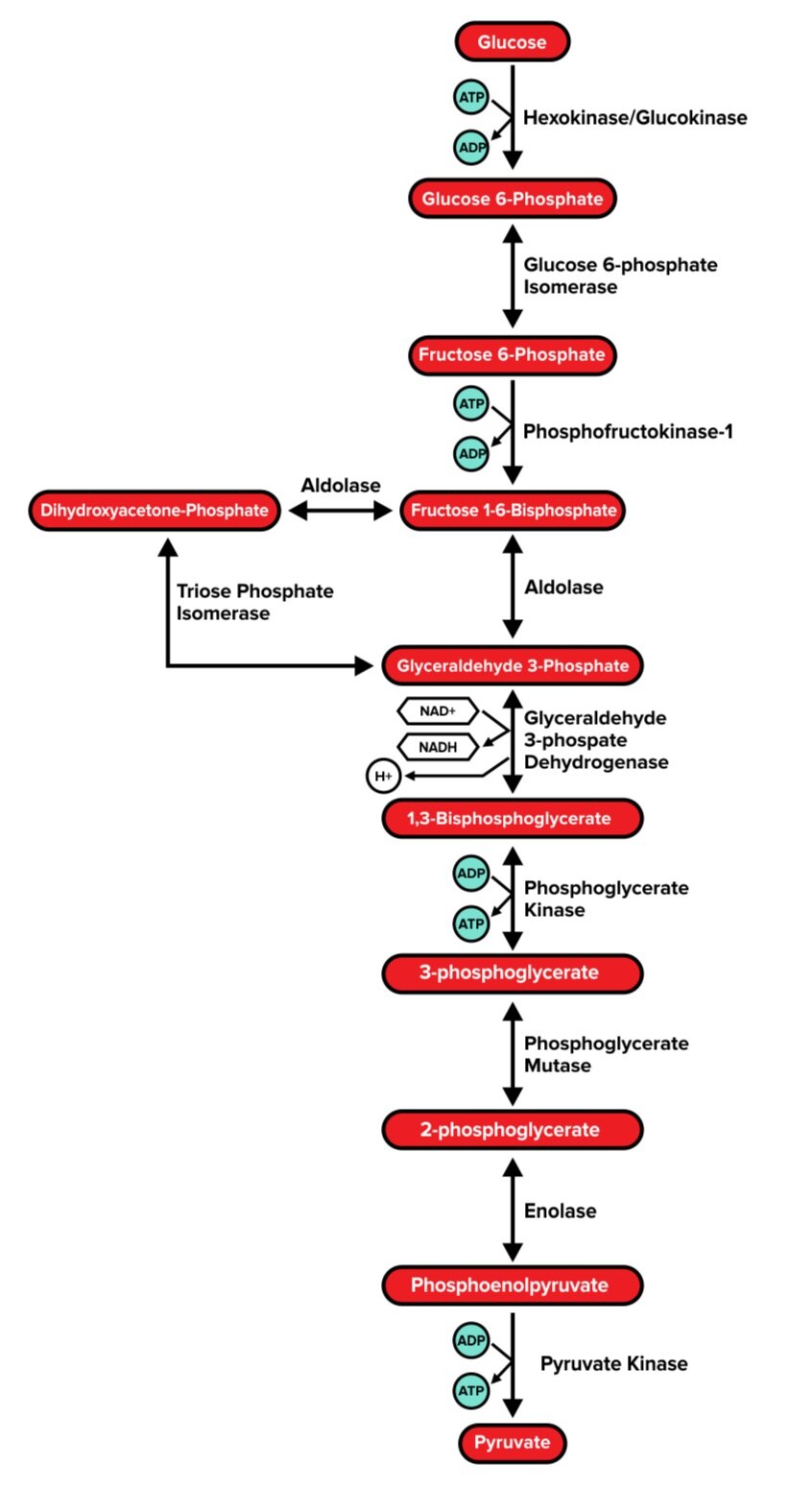
Step 1: Hexokinase/Glucokinase
Glucokinase is found in hepatocytes (liver cells) and pancreatic β-islet cells. It is activated by insulin. Hexokinase, on the other hand, is a bit more universal and found in most tissues. Both enzymes serve the same function: to use ATP to catalyze the irreversible phosphorylation of glucose.
The product of this reaction, glucose 6-phosphate, is now unable to spontaneously diffuse out of the cell. Glucose 6-phosphate also has an inhibitory effect on the hexokinase enzyme.
Step 3: Phosphofructokinase 1 (PFK-1)
Phosphofructokinase 1, also known as PFK-1, catalyzes the rate-limiting step of glycolysis. It uses ATP to catalyze the irreversible conversion of fructose 6-phosphate into fructose 1,6-bisphosphate. This step is highly regulated. Citrate (a metabolic product of aerobic respiration) and ATP have a negative feedback effect on PFK-1.
Why would this be? The presence of citrate and/or ATP indicates that the cell’s energy needs are being met, and thus signals that the glycolysis pathway is not immediately needed. Since this step is an irreversible conversion—and thus requires energy to be performed—shutting down PFK-1 when it is not needed allows the cell to conserve valuable energy.
On the other hand, the presence of AMP (adenosine monophosphate) indicates low energy in the cell and activates PFK-1.
Step 6: G3P dehydrogenase
G3P dehydrogenase catalyzes the reversible conversion of glyceraldehyde 3-phosphate into 1,3-bisphosphoglycerate, which generates one molecule NADH. However, one molecule of glucose (a 6-carbon structure) generates 2 molecules of glyceraldehyde 3-phosphate—so this step yields two molecules of NADH per glucose molecule.
Step 7: Phosphoglycerate kinase
Phosphoglycerate kinase catalyzes the reversible conversion of 1,3-bisphosphoglycerate into 3-phosphoglycerate, or the removal of a phosphate group from 1,3-bisphosphoglycerate. This generates one ATP per molecule of phosphoglycerate (or 2 ATP per glucose molecule).
Step 10: Pyruvate kinase
The final enzyme of glycolysis, pyruvate kinase, catalyzes the irreversible conversion of phosphoenolpyruvate into pyruvate, or the removal of a phosphate group from phosphoenolpyruvate. This generates one ATP per molecule of phosphoenolpyruvate (or 2 ATP per glucose molecule).
b) Lactic acid fermentation
Under anaerobic conditions, or when there is a lack of oxygen, the pyruvate molecules generated by glycolysis will undergo fermentation. During this process, lactate dehydrogenase catalyzes the conversion of pyruvate into lactate (another 3-carbon molecule) and generates NAD⁺ as a byproduct. Since it is the only enzyme in the process, it is the rate-determining step.
Why would our cells perform lactic acid fermentation, if it does not yield any ATP? The primary purpose of lactic acid fermentation is to replenish the NAD⁺ that was converted into NADH during glycolysis by glyceraldehyde 3-phosphate dehydrogenase. This makes additional NAD⁺ available to glycolytic enzymes, so our cells can continue producing 2 ATP at a time through glycolysis.
Lactic acid fermentation is part of a larger pathway known as the lactic acid cycle, or Cori cycle. Lactate generated by the muscles is sent to the liver through the bloodstream. The liver has specialized enzymes that can convert lactate into glucose, which is then sent back to the muscles.
Principles of metabolic regulation for the MCAT

The principles of metabolic regulation are covered in the Biological and Biochemical Foundations of Living Systems section of the MCAT. To prepare effectively for the exam, it’s essential that you have a clear understanding of the topic and can answer questions related to it. This guide will provide a comprehensive overview of the principles of metabolic regulation as detailed in the MCAT syllabus. This includes a breakdown of the following:
- Regulation of metabolic pathways
- Regulation of glycolysis and gluconeogenesis
- Metabolism of glycogen
- Regulation of glycogen synthesis and breakdown
- Analysis of metabolic control
Regulation of metabolic pathways
Metabolic pathways are controlled in various ways, with them all being grouped under the term metabolic regulation. Regulation tends to be seen at the beginning of pathways, rate limiting steps in a pathway (the slowest step in a pathway, that determines the rate of reaction) and irreversible reactions.
There are two ways that pathways can be regulated and positive feedback leads to the amplification of a certain output. If this output then causes further amplification, for example by activating more enzymes, then this process would be classed as a positive feedback loop. You can also have negative feedback which results in a certain output being decreased and again if this is induced by reactants/products of the pathway in question, then a negative feedback loop would be formed.
A requirement for something to be classed as living is for it to be able to perform homeostasis, this is where a dynamic steady state is maintained through the regulation of metabolic pathways. A dynamic steady state is important to keep the internal environment of an organism at a constant level, also known as equilibrium, whereby quantities of all substances remain constant whilst reactions are still taking place.
Regulation of glycolysis and gluconeogenesis
Glycolysis is a metabolic pathway that splits glucose into two pyruvate molecules without the use of oxygen (thus can be seen in both aerobic and anaerobic respiration). The rate limiting enzymes of this pathway are hexokinase, phosphofructokinase and pyruvate kinase. The purpose of glycolysis is to turn glucose into pyruvate so it can enter the TCA (also known as Krebs or citric acid) cycle to eventually generate ATP (energy).
Gluconeogenesis is a metabolic pathway that is almost the reverse of glycolysis as it generates glucose from non-carbohydrate carbon like pyruvate, lactate etc. This process is also anaerobic. The rate limiting enzymes in this process are fructose-1,6-bisphosphatase, PEP carboxylase and pyruvate carboxylase. The purpose of gluconeogenesis is to provide glucose to the body when there is not enough glucose being dietarily consumed.


Glycolysis and gluconeogenesis are controlled by the level of ATP and ADP in the body to determine whether more glucose needs to be broken down in order to supply the body with sufficient energy. When there are high levels of ATP and low levels of ADP there is no need to break down more glucose and therefore you can store it by inhibiting glycolysis and activating gluconeogenesis. When there are high levels of ADP and low levels of ATP, glucose needs to be broken down to obtain more ATP and thus glycolysis is activated and gluconeogenesis is inhibited.
There are many molecules that are used to regulate the levels of glycolysis and gluconeogenesis in the body. Glucagon decreases glycolysis and increases gluconeogenesis, overall increasing blood sugar levels. Insulin, however, causes an increase in glycolysis and a decrease in glucagon thus a decrease in blood sugar levels. Epinephrine elevates in periods of stress (where the fight or flight response is activated) and causes an increase in muscle glycolysis as well as an increase in gluconeogenesis, overall increasing blood sugar levels. Fructose-2,6-bisphosphate activates glycolysis and inhibits gluconeogenesis, overall promoting the breakdown of glucose.
Metabolism of glycogen
Glycogen can be broken down with glucose-6-phosphate which can then feed into glycolysis, the pentose phosphate pathway or convert to glucose using glucose-6-phosphatase.
- Glycogen breaks down into glucose-1-phosphate using the enzyme glycogen phosphorylase
- Glucose-1-phosphate is then turned into glucose-6-phosphate using the enzyme phosphoglucomutase
- Glucose-6-phosphate can then be used in the various pathways mentioned above
Regulation of glycogen synthesis and breakdown
Glycogen metabolism can be regulated either hormonally or allosterically. For example, epinephrine and glucagon are hormones that can promote glycogen breakdown. Hormones can also create a cAMP cascade which would lead to allosteric effects.
Cyclic AMP (cAMP) activates protein kinase A which promotes glycogen breakdown and inhibits glycogen synthesis by triggering a series of phosphorylations. Low levels of cAMP activate phosphorylases, activating glycogen synthase (needed in glycogen synthesis) and inhibiting glycogen phosphorylase (needed in glycogen breakdown).
Analysis of metabolic control
In order to find out what type of regulation a specific pathway is under you need to identify the steps of that pathway that control it. You can do this by altering different variables of the certain pathway and then recording any differences that are seen. You can quantify these differences into measurements (depending on what variable you have changed) and it allows you to generate specific mathematical models. Some examples of variables that can be changed include the abundance of metabolite or the concentration/activity of enzymes.
Hopefully this has provided you with a good understanding of the principles of metabolic regulation for the MCAT. If you’re looking for more useful revision tools, we cover a wide range of MCAT topics in our blogs, including the metabolism of fatty acids and proteins.
References
- Chaudhry, R. and Varacallo, M. (2022) ‘Biochemistry, Glycolysis’, in StatPearls. Treasure Island (FL): StatPearls Publishing.
- Edgerton, D.S. et al. (2009) ‘Effects of Insulin on the Metabolic Control of Hepatic Gluconeogenesis In Vivo’, Diabetes, 58(12), pp. 2766–2775.
- Feher, J. (2017) ‘2.9 – ATP Production I: Glycolysis’, in Feher, J. (ed.) Quantitative Human Physiology (Second Edition). Boston: Academic Press, pp. 218–226.
- Martin-Requero, A., Ayuso, M.S. and Parrilla, R. (1986) ‘Rate-limiting steps for hepatic gluconeogenesis. Mechanism of oxamate inhibition of mitochondrial pyruvate metabolism.’, Journal of Biological Chemistry, 261(30), pp. 13973–13978
- Patel, M.S. and Harris, R.A. (2016) ‘Metabolic Regulation’, in Bradshaw, R.A. and Stahl, P.D. (eds) Encyclopedia of Cell Biology. Waltham: Academic Press, pp. 288–297.
- Sherwin, R.S. et al. (1980) ‘Epinephrine and the regulation of glucose metabolism: effect of diabetes and hormonal interactions’, Metabolism: Clinical and Experimental, 29(11 Suppl 1), pp. 1146–1154.









