Physical Address
4. Cholesterol-derived hormones are known as steroid hormones. Steroid hormones can pass through the cell membrane, binding within the cell to nuclear receptors and affecting gene transcription. This allows them to exert slow-onset, long-lasting effects.
Lipids on the MCAT
Of the four main categories of biomolecules (proteins, carbohydrates, nucleic acids, and lipids), lipids often fall between the cracks. This is understandable, because proteins are absolutely essential as the building blocks of the body (and even their components, amino acids, are the highest-yield biochemistry topic for the MCAT), carbohydrate metabolism is key for understanding how cells produce energy, and nucleic acids are how genetic material is stored. Next to biological standouts like these, it’s no surprise that lipids can be easy to overlook!
That said, lipids are tested on the MCAT, which is we cover them extensively in our online MCAT Course and with private MCAT tutors. You should absolutely expect to see at least a few questions testing them directly, and maybe even a few more where they’re useful background information. In this blog post, we’ll cover some of the general expectations that the MCAT has for your lipid knowledge and point out some sub-topics worth paying close attention to.
As is always the case for biomolecules, the two overarching themes to focus on are structure and function .
Lipid Structure
In terms of structure, there are four main categories to be familiar with: (1) fatty acids and fatty acid derivatives, (2) cholesterol and its derivatives, (3) eicosanoids , and (4) terpenes and terpenoids.
Fatty Acids
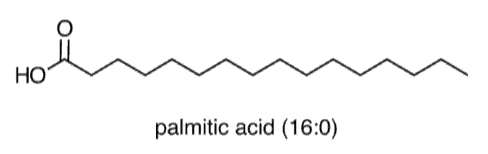
Fatty acids, cholesterol, and their derivatives are “frequent fliers” on the MCAT—that is, they are core content that you should be closely familiar with. Fatty acids , as exemplified below by palmitic acid, have a polar carboxylic acid head and a long, hydrophobic tail. They can be saturated (as is the case for palmitic acid), meaning that their hydrocarbon tail only contains single bonds, or they can be unsaturated, meaning that at least one double bond is present. As shown below, palmitic acid can be described as (16:0), which is a type of notation that indicates that it has 16 carbons and 0 C=C double bonds.
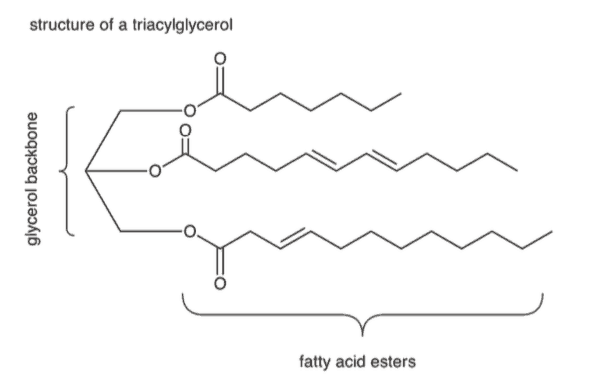
A molecule of glycerol (a three-carbon structure with three –OH groups) can form three ester bonds with fatty acids, resulting in structures known as triacylglycerols, or triglycerides, as shown below. Triacylglycerols can be modified to form structures like phospholipids (with two fatty acid chains and a phosphate group), which are the major component of the plasma membrane.
Cholesterol
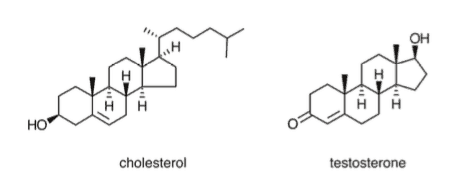
Cholesterol is a crucial component of the plasma membrane and is the basis from which several important hormones (known as steroids) are synthesized. Cholesterol and its derivatives, such as testosterone, have a characteristic four-ring structure that you can use to identify them automatically.
Eicosanoids
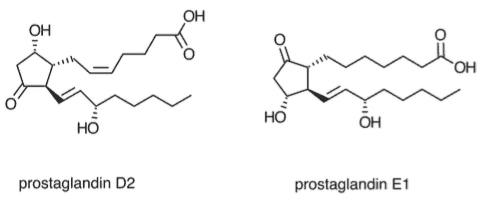
Eicosanoids are 20-carbon signaling molecules that have a characteristic 5-carbon ring flanked by long lipid chains. Prostaglandins, a category of eicosanoids, play a crucial role in modulating inflammation. You can immediately recognize them by their characteristic shape:
Terpenes
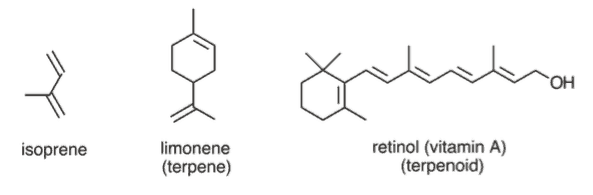
Finally, terpenes are composed of repeating isoprene (C 5 H 8 ) units. Many biologically important compounds can be described as terpenes and terpenoids. The MCAT does not expect you to know them thoroughly, but you should be able to recognize the basic pattern.
Lipid Functions
In the above concise description of the different structures of lipids, we briefly touched on some of their functions . The following functions of lipids are especially important to familiarize yourself with for Test Day:
1. Triacylglycerols and fatty acids are a source of energy that can be stored and released through beta-oxidation, which is a highly productive form of energy metabolism. (In fact, this is why we use fats for long-term energy storage in the body).
2. Phospholipids (modified triglyceride derivatives) are the major underlying structural component of the bilayer plasma membrane of the cell.
3. Cholesterol in the cell membrane modulates its fluidity, increasing fluidity at low temperatures and decreasing it at high temperatures.
4. Cholesterol-derived hormones are known as steroid hormones. Steroid hormones can pass through the cell membrane, binding within the cell to nuclear receptors and affecting gene transcription. This allows them to exert slow-onset, long-lasting effects.
Most of the key functions of lipids turn out to be related to their structure. All lipids have a considerable nonpolar component. This is why hydrophobic molecules can also sometimes be described as lipophilic (= “loving lipids”). Additionally, lipids may have a polar area, making them “amphipathic” (a term used to describe molecules with both polar and nonpolar areas). The amphipathic properties of phospholipids is what allows them to form the bilayer plasma membrane effectively, and the nonpolar nature of steroid hormones is what allows them to pass through that plasma membrane. In contrast, large and polar peptide hormones must interact with the cell via membrane-bound receptors. This is a classic example for the MCAT of how low-level structural properties affect high-level physiological dynamics, and as you study, be sure to keep an eye out for examples like this!
This is far from a comprehensive overview of lipids (an entire chapter of biochemistry is needed for that), but hopefully it has given you a sense of where to start and how to organize your studying. If you’re just getting started with your prep, Next Step offers a free MCAT practice bundle that includes a half-length diagnostic, access to our first full-length practice test, and a demo of our online course. You can sign up for the free practice bundle here . If you’re looking for more comprehensive prep, we also offer one-on-one tutoring programs as well as an online MCAT course . Not sure where to start? Set up a free consultation with one of our experienced Academic Managers. They will go over your prep needs and help you decide what prep options are right for you.
Written by Blueprint MCAT (formerly Next Step Test Prep) MCAT experts.
MCAT is a registered trademark of the Association of American Medical Colleges (AAMC), which is not affiliated with Blueprint.
Lipids and Membranes for the MCAT: Everything You Need to Know
Learn key MCAT concepts about lipids and membranes, plus practice questions and answers

(Note: This guide is part of our MCAT Biochemistry series .)
Table of Contents
Part 1: Introduction to lipids and membranes
Part 2: Lipid structures
a) Insolubility of lipids
b) Signaling lipids
c) Structural lipids
Part 3: Cell membranes and components
a) Phospholipid bilayers
b) The fluid mosaic model
c) Major components of the cell membrane
d) Transporters
Part 4: High-yield terms
Part 5: Passage-based questions and answers
Part 6: Standalone questions and answers
Part 1: Introduction
In the early twentieth century, one of the most common therapies to control seizures in pediatric patients suffering from epilepsy was not a medication but a diet. Minimizing carbohydrates while consuming more fat seemed to alleviate seizures while still providing children the nutrients they needed to properly develop.
Fats, also known as lipids, are relatively simple molecules responsible for a variety of functions in our body, including energy storage and transmitting signals. The diversity of roles that lipids hold often makes them a challenging topic for students during their MCAT prep. Lipids are everywhere in your body—from hormones, cellular structures, and more. Indeed, they are a likely topic to show up on your exam.
In this guide, we will explain everything you need to know about lipids to be successful on the MCAT. At the end, we’ll include some practice passages and discrete questions so you can apply your knowledge in the exact context the AAMC will on test day.
Let’s get started!
Part 2: Lipid structures
a) Insolubility of lipids
Carbohydrates are characterized by their 1:2:1 ratio of carbon, hydrogen, and oxygen. Amino acids and proteins possess distinctive amine and carboxyl functional groups. Lipids are characterized by their hydrophobic alkyl structures and are primarily composed of carbon and hydrogen atoms.
Below we have testosterone, a signaling lipid, and a triglyceride, an energy-storing lipid.


Although they may look very different, they are both hydrophobic lipids. The hydrocarbon ring structure of testosterone and the hydrocarbon tail of the triglyceride will cause the molecules to be nonpolar and insoluble in polar solvents (such as water). This is a unifying feature of all lipids and has special implications for how lipids are used in the body.
Due to their nonpolar properties, lipids have assumed many different functions within organisms. All cell membranes are composed of lipids (as we will discuss), and many animals use lipids as a form of energy storage. Waxes are protective secretions that serve as a waterproofing compound for many plants and animals. The structure of waxes is characterized by long, alkane chains.
b) Signaling lipids
Signaling lipids are specialized lipids involved in signal transduction pathways, the passing of information between and within cells. These signaling lipids are further divided into two categories: steroids and fat-soluble vitamins.
Steroids are characterized by their four-ring structure, which includes three cyclohexanes and one cyclopentane. As you’d expect, these hydrocarbon rings make steroids nonpolar. The most commonly tested steroid on the MCAT, cholesterol, is one you should be very familiar with. It plays an integral role in our cell membranes (more on this later) and is a precursor for many molecules, including steroid hormones. Steroid hormones are specially secreted by endocrine glands and act as hormones. You can find more information about steroid hormones in our guide on the endocrine system.

Testosterone, the hormone we mentioned earlier, is just one of the many steroid hormones used by your body. Take another look at testosterone’s structure and see if you can identify the rings that characterize steroids.
Terpenes and terpenoids are aromatic secretions following the formula (C₅H₈)₁₁. Terpenes are typically rich in double bonds between carbons, allowing these molecules to undergo cyclization reactions. Squalene is an especially important terpene molecule as it is the biological precursor of steroids in the human body, including cholesterol.
Prostaglandins are an additional class of hormones derived from lipids. In contrast to steroid hormones, which often use cholesterol as a precursor, prostaglandins are derived from arachidonic acid. Prostaglandins contain at least one five-carbon ring.

Vitamins are nutrients that must be acquired through the diet. They often function as cofactors for enzymes. They can be divided into two categories: water-soluble vitamins and fat-soluble vitamins.
When in excess, water-soluble vitamins will be excreted in the urine, whereas fat-soluble vitamins will be stored in fat tissue. For the MCAT, you should know that vitamins B and C are water-soluble, while vitamins A, D, E, and K are fat-soluble.
c) Structural lipids
Like cholesterol, structural lipids play a crucial role in the cell membrane. These lipids are amphipathic, which simply means they have hydrophobic and hydrophilic regions on the same molecule. Their hydrophobic region is a nonpolar tail (typically composed of alkyl hydrocarbons), while the hydrophilic region is a polar head (such as a phosphate or glycerol group).

You may recall similar-sounding words with quite different meanings. For instance, an amphoteric compound can react as an acid or a base, and an amphiprotic substance can donate or receive a proton. It’s important not to get these terms confused!
For the MCAT, the two main structural lipids you should be familiar with are phospholipids and sphingolipids.
Phospholipids, or phosphatides, are the primary component of the phospholipid bilayer of the cell membrane. They contain a hydrophilic, polar phosphate head group joined through an ester linkage to a hydrophobic nonpolar fatty acid tail. An important property to keep in mind of these lipids is their degree of saturation. In this context, saturation refers to the number of single bonds a carbon atom has with other molecules.
Saturated fatty acid tails only have single bonds, so every carbon atom is bonded to four other atoms. These fully saturated tails form van der Waals interactions with other saturated fatty acid tails around them. Due to the symmetric, orderly nature of these alkyl chains, they can more easily form a cohesive structure and tend to be solid at room temperature.

Unsaturated fatty acid tails, on the other hand, contain one or more double bonds. These double bonds introduce “kinks” in the structure of the alkyl chain. This makes them less likely to stack and solidify. Thus, at room temperature, unsaturated fatty acids tend to be found in liquid form.
Why is this distinction important? As we will later discuss, the saturation of these fatty acid tails impacts the fluidity of the cell membrane. In general, unsaturated fatty acids are more prevalent in fluid regions of the cell membrane compared to saturated fatty acids.
Glycerophospholipids are an important type of phospholipids. Instead of a single phosphate head group, these contain a glycerol backbone that forms ester linkages to two fatty acids and a polar head group. Sphingolipids have a similar structure to glycerophospholipids—but instead of glycerol, they contain a sphingosine backbone in addition to the typical polar head group and nonpolar fatty acid tail.
Triglycerides, also known as triacylglycerols, are an additional group of lipids, which are composed of a glycerol head group and three fatty acid tails. They are primarily used for energy storage in specialized cells called adipocytes, and feature prominently in metabolism.









