Physical Address
B) N-terminal Ala6 tag
Free MCAT Practice Questions

Below are 10 practice questions to help you study for the Biology & Biochem portion of the MCAT exam. Part 1: Biological and Biochemical Foundations of Living Systems Use the following passage to answer questions 1 through 4. Hospitals and clinics in the United States and around the world face constant shortages in the supply of blood for transfusions. One alternative currently being explored is to make recombinant human hemoglobin (rHb). In addition to increasing supply for transfusions, rHb has a longer shelf life than red blood cells, is compatible with all blood types, and poses lower risk of transmitting disease. Despite these advantages, neither rHb products nor hemoglobin purified from animals has been licensed as therapy in the United States, mainly because of reports of hypertension and associated cardiovascular and cerebrovascular problems. (A product called HBOC-201, which is purified from cows, has been approved for human use in South Africa and Russia.) The toxicity of rHb has been attributed to the protein’s presence in the plasma, where it can scavenge nitric oxide (NO), undergo oxidative reactions that damage tissues, denature, aggregate, and precipitate, among other activities. In contrast, endogenous hemoglobin localizes in erythrocytes, where the cell membrane and cellular reduction pathways reduce or prevent these activities. It has been proposed that the hypertensive effects of rHb could be due to the protein having too low an affinity for oxygen, causing excessive release of oxygen into the arterioles and vasoconstriction. Alternatively, it has been proposed that NO scavenging by rHb from the vasculature leads to depletion of NO, an increase in intracellular calcium, and muscle constriction. Since the 1980s when researchers started studying rHb, various conjugates and amino acid substitutions have been tested for the ability to reduce issues with stability and toxicity. Through mutagenesis strategies, it has been possible to identify rHb variants with different rates of oxygen binding and release, as well as some that are not associated with increases in blood pressure or mean arterial pressure (MAP). However, more work is needed to identify variants with reduced loss of hemin (a protoporphyrin ring containing chelated iron) and globin denaturation. Work is also underway to lower production costs of rHb variants that are being developed as potential therapeutic products. 1. The table summarizes the difference in blood pressure associated with six variants of rHb along with several parameters in vitro.  Based on the data in the table, which parameter is most likely to be responsible for the increase in MAP observed for some rHb variants? a. NO is being scavenged and depleted.
Based on the data in the table, which parameter is most likely to be responsible for the increase in MAP observed for some rHb variants? a. NO is being scavenged and depleted.
b. Too much oxygen is being released.
c. The affinity for oxygen is too high.
d. rHb is unstable and autoxidation is occurring. 2. In the figure below, the four curves represent rHb variants harboring point mutations and their oxygen-binding curves.  Which curve is most similar to the binding curve of endogenous hemoglobin, and why? a. The curve for α(L29F)β(WT) because hemoglobin has maximal affinity for oxygen at low and high concentrations.
Which curve is most similar to the binding curve of endogenous hemoglobin, and why? a. The curve for α(L29F)β(WT) because hemoglobin has maximal affinity for oxygen at low and high concentrations.
b. The curve for α(V96W)β(WT) because hemoglobin has high affinity for oxygen at low and high concentrations.
c. The curve for α(L29F/V96W)β(N108K) because hemoglobin has low affinity for oxygen at low concentrations and high affinity for oxygen at high concentrations.
d. The curve for α(V96W)β(N108K) because hemoglobin has low affinity for oxygen at low and high concentrations. 3. Engineering a cellular rHBOC involves recombinant DNA technology. What is necessary to make recombinant hemoglobin? a. Expression of hemoglobin protein in bacteria
b. Knowing the sequence of the hemoglobin gene
c. Knowing the hemoglobin gene’s splice donor and acceptor sites
d. Having the crystal structure of hemoglobin protein 4. Which condition would NOT be expected to result in increases in extracellular hemoglobin? a. Liver cirrhosis
b. Blood transfusion
c. Inflammatory bowel disease
d. Malaria infection 5. Which of the following amino acids does NOT have an isoelectric point (pI) between 5.5 and 6.0? a. Alanine
b. Glutamic acid
c. Glycine
d. Glutamine 6. Which amino acid would most likely be found on the surface of a protein molecule at physiological pH? a. Isoleucine
b. Lysine
c. Alanine
d. Proline 7. Which of the following statements about terpenes is NOT true? a. They are a type of terpenoid.
b. They all contain double bonds.
c. They are all made up of 5-carbon units.
d. They all contain oxygen. 8. How are the plasma membranes of mammalian and bacterial cells similar? a. They typically contain cholesterol.
b. They have negatively charged lipids on their surfaces.
c. They contain lipids that are involved in signal transduction.
d. They are made up of many different types of phospholipids. Use the following passage to answer questions 9 and 10. Under conditions of cell stress, such as exposure to heat, the weak bonds within a protein can be broken, leading to protein misfolding and self-association. When the concentration of misfolded polypeptides becomes high enough, they can form larger aggregates that are very stable because strong bonds occur between the molecules. Many age-related diseases, including Alzheimer’s, Parkinson’s, and type 2 diabetes, are considered to be the result of protein aggregates, which can eventually cause tissue death. Chaperone molecules bind with high affinity to exposed hydrophobic regions on the surface of misfolded polypeptides and reduce aggregation. However, there is increasing evidence that chaperones do not merely act like sponges that associate with misfolded polypeptides and prevent them from aggregating. Along with a complex of molecules, chaperones can help misfolded substrates unfold and are thus also called unfoldases. Once the substrates are completely unfolded, they can then spontaneously refold into their native conformation. Although chaperones can act on different types of proteins, they unfold them with varying degrees of efficiency. For example, one of the major families of chaperones, which are highly conserved from bacteria to eukaryotes, is called GroEL/GroES. Compared with other chaperones, GroEL/GroES can rapidly convert misfolded rhodanese (rho), a mitochondrial enzyme involved in detoxifying cyanide, into its unfolded form. However, it is unclear in general whether a vast excess of native, properly folded proteins could compete with misfolded substrates for binding to chaperones. Indeed, native proteins are present in a cell at a much higher concentration relative to misfolded species. Furthermore, if chaperones bind inappropriately to substrates that they are not able to completely unfold, the chaperone molecules may not dissociate rapidly from the partially unfolded species and their activity could get stalled. Another area of question is the role of ATP in the unfolding and refolding of polypeptide substrates. When GroEL/GroES is bound to substrate, ATP hydrolysis leads to conformational changes in GroEL and the release of GroES from the complex. One model is that the ATP is not required for the binding of chaperones to misfolded polypeptides but that, at least for some chaperones, ATP hydrolysis is required for the release of the unfolded polypeptide from the chaperone complex so it can refold in solution. 9. The graph below shows the concentration of rhodanese (Rho) that is refolded into its native confirmation in the presence (solid shapes) or absence (open shapes) of an excess concentration of native protein, called MDH. Rhodanese was also incubated with the chaperone GroEL/GroES (LS, ELS) (top four curves) or without (bottom two curves) and with ATP (top two curves) or without (bottom four curves).  Based on the data presented in the graph, how does native MDH affect the refolding of rhodanese by the chaperone molecules? a. The refolding is not affected because the chaperones bind MDH and Rho simultaneously.
Based on the data presented in the graph, how does native MDH affect the refolding of rhodanese by the chaperone molecules? a. The refolding is not affected because the chaperones bind MDH and Rho simultaneously.
b. The refolding rate is reduced but the yield of refolding is not changed.
c. The refolding does not occur because MDH stalls the chaperones.
d. The refolding yield is reduced because MDH depletes ATP. 10. Which of the following is NOT a chaperone molecule? a. Heat-shock protein (Hsp) 70
b. SecB
c. GroEL/GroES
d. PI3K
Download Answer Key
Enter your email address below to download our full MCAT practice question PDF document along with the answer key to see how well you know your biology and biochemistry before test day.
MCAT Biochemistry Practice Questions
Prepare for the MCAT Biochemistry section with practice questions

Introduction
MCAT Biochemistry Practice Passage #1
MCAT Biochemistry Practice Passage #2
MCAT Biochemistry Practice Passage #3
MCAT Biochemistry Practice Questions (Standalone)
Introduction
You are ready to sit down and study for the MCAT, which is one of the most important pieces of your medical school application. Achieving a great MCAT score can greatly increase your chances of hearing “We’re pleased to inform you…” from the medical school of your dreams.
Your test date looms in the future, and you’re in the thick of your test preparation. You’ve created a perfect study schedule and have started diving into your content review. But, you find that content review is not enough to score well on the MCAT! You need to be able to apply your content knowledge to the rest of the exam.
Biochemistry is one of the most heavily tested subjects on the MCAT. From enzymes to experimental techniques (link experimental techniques page), biochemistry questions will make up a portion of both your chemistry/physics and biology/biochemistry section scores.
Use the following three passages and five standalone questions to test your ability to apply your biochemistry knowledge to real, MCAT-style passages. You’ll notice that each explanation has suggestions for what you should review if you miss a question. Let’s get started, and good luck!
MCAT Biochemistry Practice Passage #1
Nuclear estrogen receptor 2 (ERB2) suppresses tumor growth and modulates cancer cell proliferation in breast cancer (BC). Loss of ERB2 is shown to correlate with early stages of ductal and breast tumors, though the specific mechanism is largely unknown. Researchers hypothesized that identifying and characterizing multiprotein complexes involved in ERB2 function might identify the molecular basis for the role of protein in BC.
Using interaction proteomics, which combines native protein complex purification and mass spectroscopy, it was determined that ERB2 interacts with other proteins in BC cells, and this interaction is mediated by one or more RNAs.
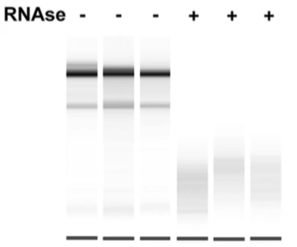
To probe the effect of RNA on ERB2 complex formation, researchers treated a nuclear extract containing ERB2 and its putative binding partners with RNase. The results from the experiment are shown in Figure 1.
Next, the researchers analyzed ERB2 complex formation with putative binding partner AGO2 in the absence and presence of RNase. The results are shown in Figure 2. The researchers analyzed the nuclear extract (input), the amount of bound AGO2, and two TEV IgG-Sepharose column eluents using a Western blot for AGO2.
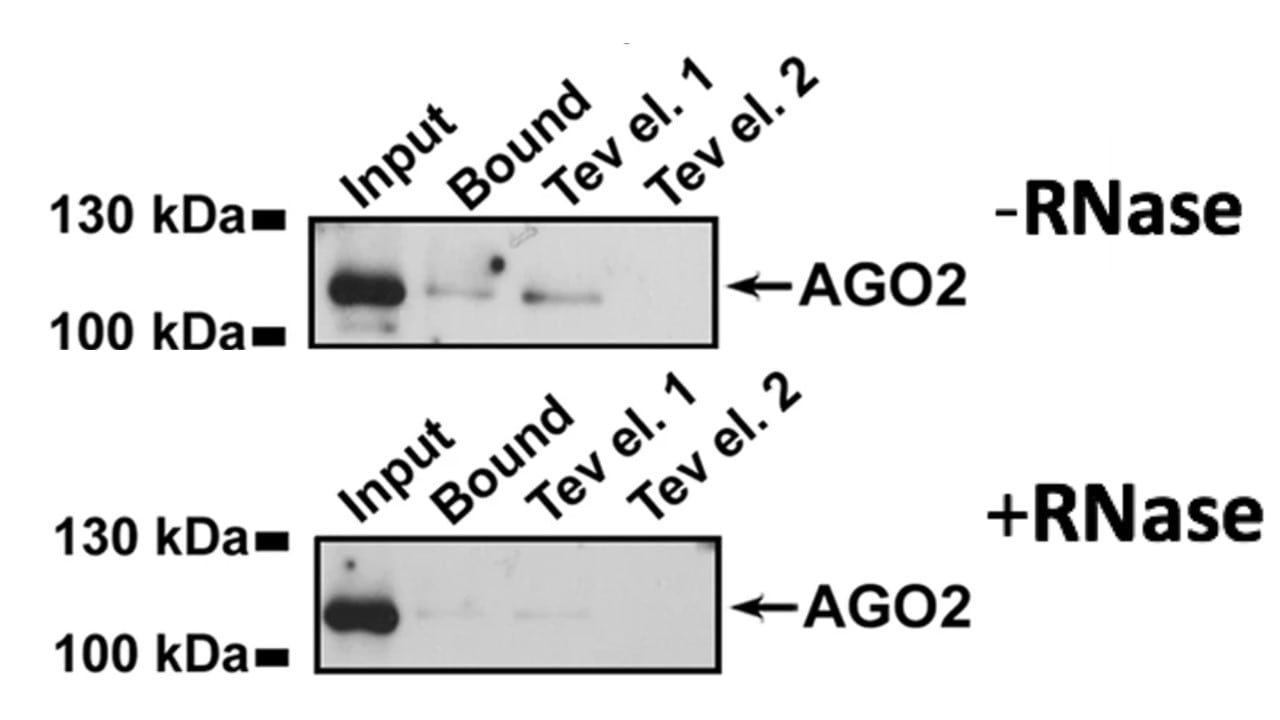
CREATOR AND ATTRIBUTION PARTY: GIURATO, G., NASSA, G., SALVATI, A., ET AL. QUANTITATIVE MAPPING OF RNA-MEDIATED NUCLEAR ESTROGEN RECEPTOR B INTERACTOME IN HUMAN BREAST CANCER CELLS. SCI DATA 5, 180031 (2018). THE ARTICLE’S FULL TEXT IS AVAILABLE HERE: https://www.nature.com/articles/sdata201831. THE ARTICLE IS NOT COPYRIGHTED BY SHEMMASSIAN ACADEMIC CONSULTING. DISCLAIMER: SHEMMASSIAN ACADEMIC CONSULTING DOES NOT OWN THE PASSAGE PRESENTED HERE. CREATIVE COMMON LICENSE: HTTP://CREATIVECOMMONS.ORG/LICENSES/BY/4.0/. CHANGES WERE MADE TO ORIGINAL ARTICLE TO CREATE AN MCAT-STYLE PASSAGE.
1. Which of the following most likely contributes the greatest amount stability to the ERB2-AGO2 interaction?
A) Disulfide bridges
B) Hydrogen bonding
C) Hydrophobic effect
2. Researchers decide to purify ERB2 using a nickel column. They most likely prepare ERB2 with a:
A) C-terminal His6 tag
B) N-terminal Ala6 tag
C) C-terminal Glu8 tag
D) N-terminal Val8 tag
3. To interpret the results of Figure 1, the researchers must assume all of the following EXCEPT:
A) Equal concentrations of RNase were used in the RNase + lanes.
B) Equal concentrations of nuclear extract were loaded in all of the lanes.
C) Equal amounts of ERB in complex with other proteins form in all of the lanes after RNase treatment.
D) The antibody for ERB is specific to the protein at a site distinct from protein interaction sites.
4. Why do researchers include the input lanes in both RNase conditions in Figure 2?
A) To determine the concentration of AGO2 before the experiment
B) To ensure that equal amounts of AGO2 were present before the experimental treatment
C) To measure the amount of ERB2 complex without RNase
D) To increase the likelihood of binding events for later conditions
5. Which of the following conclusions is best supported by Figure 2?
A) An RNA molecule plays a role in the AGO2-ERB2 interaction
B) ERB2 is not expressed when RNase is present
C) AGO2 binds ERB2 more tightly in the absence of RNA
D) AGO2 is upregulated when RNase is present
Answer key for practice passage #1
1. The correct answer is C. The hydrophobic effect provides the most energy stabilization for interactions between proteins (choice C is correct). Disulfide and salt bridges are formed within a protein and not between two different proteins (choices A and D are incorrect). Hydrogen bonding contributes weakly, if at all (choice B is incorrect).
2. The correct answer is A. His6 tags are added to proteins (at either the N- or C-terminus) to allow the tagged proteins to bind to columns (choice A is correct). The other tags are generally not used in experiments, and they would not be used for a nickel column as His-tags are standard.
Review protein purification techniques and different types of purification columns (i.e. cation-anion exchange, size exclusion, nickel, etc.)
3. The correct answer is C. The western blot provides information on whether or not the ERB2 protein is bound to another protein. When ERB2 runs higher, that means it is bound to another protein as it migrates more slowly through the gel. When ERB2 runs lower, that means it is unbound. Since the ERB2 is seen at different levels in the gel, the researchers cannot assume that equal amounts of ERB2 are in the complex after treatment (choice C is correct). Equal RNase should be used as a control (choice A is incorrect). Equal nuclear extract should be used as a control (choice B is incorrect). In order for the ERB2 antibody to work, it must be able to bind the protein, and it cannot bind if another protein is in the way (choice D is incorrect).
Review Western blotting technique and use of antibodies in molecular biology.
4. The correct answer is B. This lane is a control to ensure that later treatments are not the result of starting with different amounts of material. Since AGO2 is being measured here (as indicated in the figure caption), choice B is correct. Western blots do not provide information about the concentration of protein (choice A is incorrect). AGO2 is being targeted, not ERB2 (choice C is incorrect). Measuring the control will not increase the likelihood for binding events at later experimental stages (choice D is incorrect).
Review Western blotting technique.
5. The correct answer is A. From Figure 2, it is observed that the amount of AGO2 bound to ERB2 decreases after RNase treatment. Therefore, it is reasonable to conclude that an RNA molecule is involved in the AGO2-ERB2 interaction (choice A is correct; choice C is incorrect). ERB2 is not being measured in the Western blot presented in Figure 2 (choice B is incorrect). The input level of AGO2 is the same with or without the RNase, so AGO2 is not upregulated when RNase is present (choice D is incorrect).
Review Western blotting technique.









