Physical Address
a) Overview of functional groups
SN1 and SN2 question

I read somewhere once that it’s theoretically possible that a good LG attached to a primary carbon can undergo SN1 if rearrangements can happen simultaneously at the same time. However, I’ve seen others present it as simply no reaction — ignoring rearrangements all together. For the purposes of the MCAT, do we regard all primary LG’s as unreactive by SN1 (with the exception of allylic or benzyllic primary LG’s)?
EDIT: Example: 1-Cyclohexyl-1-chloro-methane
The neighboring carbon is too bulky for it to undergo SN2 (its neighboring carbon blocks back-side attack). For SN1, if the LG were to fall off, it would result in a primary carbocation. Some would just say this is no reaction and call it a day. . but if you were to consider a hydride shift occurring at the same time as the LG leaving, it would place a carbocation on a tertiary carbon and make the reaction really favorable for SN1. This is sorta odd because I’m use to thinking of metyhl and hydride shifts happening after a carbocation forms, but in this instance, it can occur simultaneously — atleast according to this source (klein).
I don’t want to overthink this lol, but can someone please guide me in the right direction?! Is this one of those nuances I should disregard for the MCAT, or is this actually worth remembering?
Fundamentals of Organic Chemistry for the MCAT: Everything You Need to Know
Learn key MCAT concepts about the fundamentals of organic chemistry, plus practice questions and answers
(Note: This guide is part of our MCAT Organic Chemistry series.)
Table of Contents
Part 1: Introduction to the fundamentals of organic chemistry
Part 2: Arrow-pushing mechanisms
a) Double-headed arrows
b) Single-headed arrows
Part 3: Nucleophiles and electrophiles
a) Overview of functional groups
b) Nucleophilic substitution reactions
c) Elimination reactions
Part 4: High-yield terms
Part 5: Passage-based questions and answers
Part 6: Standalone questions and answers
Part 1: Introduction to the fundamentals of organic chemistry
At first blush, organic chemistry can be quite frightening. The topic covers many concepts: from electron movement to functional groups and organic structures. To top it off, many college-level organic chemistry courses require the memorization of complicated, multi-step reactions.
Here is some good news: the organic chemistry content tested on the MCAT may be much less extensive than what you have learned in a college class. In this guide, we will introduce the fundamental concepts of organic chemistry you must be familiar with to succeed on the MCAT. By the end of this guide, you will be well-prepared to tackle more complex organic chemistry topics in our other guides.
Throughout this guide, there are several high-yield terms listed in bold. These terms will also be listed at the end of the guide. Additionally, there are several MCAT-style practice questions for you to test your knowledge of these fundamental principles.
Let’s get started!
Part 2: Arrow-pushing mechanisms
a) Double-headed arrows
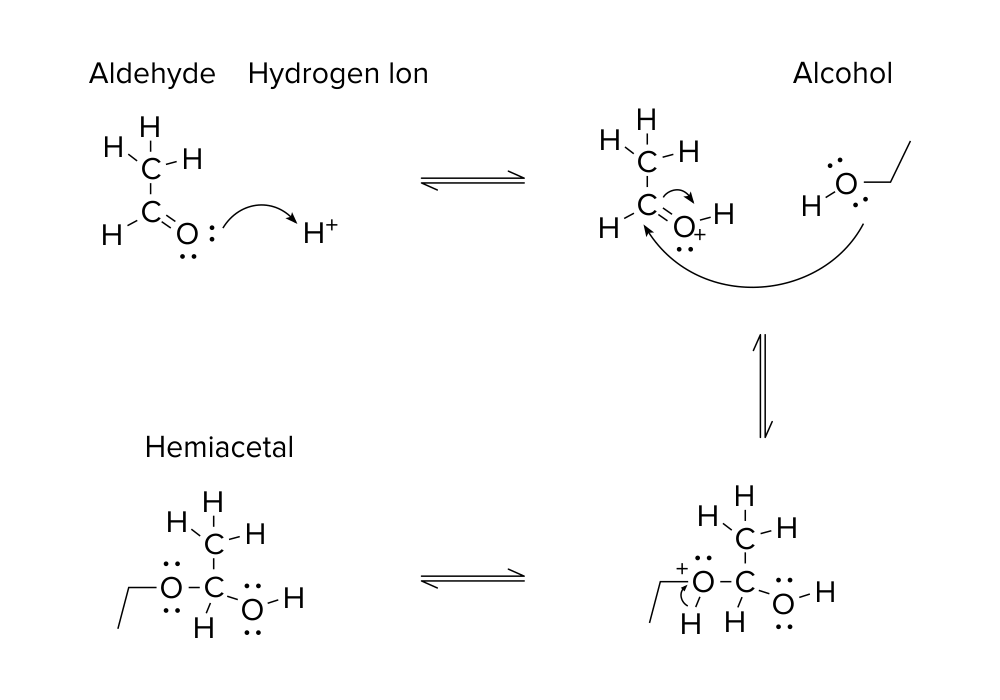
Take a look at the organic reaction above. A quick glance shows that a reaction occurs to produce a new product. A molecule with an aldehyde functional group reacts with a hydrogen ion and alcohol to produce a hemiacetal. (For more information on this topic, be sure to refer to our organic chemistry guide on functional groups.)
All of these molecules are connected by two types of arrows. There are the two half-arrows pointing to the left and to the right in between each step of the reaction. These arrows, known as equilibrium arrows, signify that a reaction is reversible. Thus, each step of the reaction can proceed to the next or previous step with equal probability. Equilibrium arrows are not necessarily always the same length. A reaction can be reversible and have equilibrium arrows of different lengths. The length of the half-arrow corresponds to the likelihood that the organic reaction will travel in that direction.
Mechanisms are illustrated depictions of chemical reactions. Mechanisms are used to illustrate the formation and breaking of various bonds between atoms. In general, mechanisms typically use arrows to depict the movement of electrons. Arrows are generally drawn with an origin at a specific electron pair and point to the bond or atom they attack. Double-headed arrows indicate the movement of an electron pair, or two electrons at once.
Take another look at the illustrated mechanism, and track the movement of electrons between each step.
- In the first step, valence electrons from the oxygen atom attack the hydrogen ion to initiate this reaction.
- In the second step, there are two double arrows. The first double arrow originates from the valence electrons on the oxygen in the alcohol group and attacks the carbon atom. The second double arrow shows the movement of electrons from the carbon-oxygen double bond to the oxygen atom.
- In the third step, electrons from the oxygen-hydrogen bond move to the oxygen, releasing a hydrogen ion.
It’s important to remember that drawing double arrows in a reverse fashion (from a source without electrons) is incorrect and can result in a costly mistake when interpreting a reaction on the MCAT! The arrows extend to the target from the source of electrons.
b) Single-headed arrows
Arrows can be double- or single-headed, with either type having a different meaning.
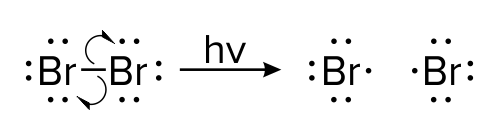
In the organic reaction shown above, a single bromine molecule (Br2) splits into two bromine ions (Br-). The catalyst for this reaction is light; in chemical reactions, the letters hv are used to represent high-energy light that breaks bonds. In contrast to the first mechanism, this mechanism uses single-headed arrows.
Single-headed arrows are similar to double arrows in that they show the movement of electrons. Like double-headed arrows, single-headed arrows extend from the source of electrons to the target. In this example, electrons originate in a single bond and are sent to each bromine atom.
Unlike double-headed arrows, single-headed arrows, also referred to as fish hooks, show the movement of only one electron. Thus, as the original bond in the bromine molecule is broken, each resulting ion receives only one electron (instead of a pair of electrons).