Physical Address
a) Ionic bonds
Coordinate Covalent Bond Mcat
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
Electrons exist outside of an atom‘s nucleus and are found in principal energy levels that contain only up to a specific number of electrons. The outermost principal energy level that contains electrons is called the valence level and contains valence electrons. Lewis symbols and formulas use dots to visually represent the valence electrons of an atom and can be used to represent covalent bonds as shared pairs of electrons between atoms. Lewis symbols do not visualize the electrons in the inner principal energy levels.
The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots per side. (It does not matter what order the positions are used.) When drawing more complex covalent compounds we need to also consider the formal charge and resonance structures of each compound.
![]()
For example, the Lewis electron dot diagram for calcium is simply:
Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:

Likewise, they can be used to show the formation of anions from atoms, as shown below for chlorine and sulfur:

When considering how atoms come together to form covalent bonds we need to consider Lewis diagrams which can illustrate how atoms come together to share pairs of electrons. Two H atoms can come together and share each of their electrons to create a covalent bond. The shared pair of electrons can be thought of as belonging to either atom and thus each atom now has two electrons in its valence level.

In many atoms, not all of the electron pairs comprising the octet are shared between atoms. These unshared, non-bonding electrons are called lone pairs of electrons. Although lone pairs are not directly involved in bond formation, they should always be shown in Lewis structures.
Lewis structures can be drawn in 4 steps:
- Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the most number of possible bonds to be the central atom).
- Draw Lewis symbols of the individual atoms in the molecule.
- Bring the atoms together in a way that places eight electrons around each atom (or two electrons for H, hydrogen) wherever possible.
- Each pair of shared electrons is a covalent bond that can be represented by a dash.
Lewis structures can also be drawn for polyatomic ions. The Lewis structure of an ion is placed in brackets and its charge is written as a superscript outside of the brackets, on the upper right. Ions are treated almost the same way as a molecule with no charge. However, the number of electrons must be adjusted to account for the net electric charge of the ion. When counting electrons, negative ions should have extra electrons placed in their Lewis structures, while positive ions should have fewer electrons than an uncharged molecule.
Although we know how many valence electrons are present in a compound, it is harder to determine around which atoms the electrons actually reside. To assist with this problem, chemists often calculate the formal charge of each atom. The formal charge is the electric charge an atom would have if all the electrons were shared equally.
The formal charge can be calculated using the formula:

For example, the formal charge of an oxygen atom in carbon dioxide is:

Sometimes multiple Lewis structures can be drawn to represent the same compound. These equivalent structures are known as resonance structures and involve the shifting of electrons and not of actual atoms. Depending on the compound, the shifting of electrons may cause a change in formal charges. Most often, Lewis structures are drawn so that the formal charge of each atom is minimized. Resonance structures have the same number of electrons and therefore have the same overall charge. Resonance structures differ only in the arrangement of electrons; the atoms keep the same connectivity and arrangement.

When you are drawing resonance structures, it is important to remember to shift only the electrons; the atoms must have the same position. Sometimes, resonance structures involve the placement of positive and negative charges on specific atoms. Because atoms with electric charges are not as stable as atoms without electric charges, these resonance structures will contribute less to the overall resonance structure than a structure with no charges.
G. N. Lewis proposed a generalized definition of acid-base behavior in which acids and bases are identified by their ability to accept or to donate a pair of electrons and form a coordinate covalent bond.
A coordinate covalent bond (or dative bond) occurs when one of the atoms in the bond provides both bonding electrons. For example, a coordinate covalent bond occurs when a water molecule combines with a hydrogen ion to form a hydronium ion. A coordinate covalent bond also results when an ammonia molecule combines with a hydrogen ion to form an ammonium ion. Both of these equations are shown here.

A Lewis acid is any species (molecule or ion) that can accept a pair of electrons, and a Lewis base is any species (molecule or ion) that can donate a pair of electrons. A Lewis acid-base reaction occurs when a base donates a pair of electrons to an acid. A Lewis acid-base adduct, a compound that contains a coordinate covalent bond between the Lewis acid and the Lewis base, is formed. The following equations illustrate the general application of the Lewis concept.
Practice Questions
Khan Academy
MCAT Official Prep (AAMC)
Chemistry Question Pack Passage 15 Question 84
Practice Exam 2 C/P Section Passage 6 Question 31
Practice Exam 2 C/P Section Passage 7 Question 36
Practice Exam 2 C/P Section Passage 7 Question 39
Practice Exam 3 C/P Section Passage 7 Question 37
Key Points
• Lewis symbols are diagrams that show the number of valence electrons of a particular element with dots that represent lone pairs.
• Lewis structures incorporate an atom’s formal charge, which is the charge on an atom in a molecule, assuming that electrons in a chemical bond are shared equally between atoms.
• Lewis dot diagrams are often employed to visualize the covalent bonding between atoms in a compound. However, when multiple equally valid structures can be drawn, these structures are called resonance structures. Resonance structures have the same number of electrons and therefore have the same overall charge.
• A Lewis acid is any species (molecule or ion) that can accept a pair of electrons, and a Lewis base is any species (molecule or ion) that can donate a pair of electrons.
Key Terms
Valence level: The outermost principal energy level, which is the level furthest away from the nucleus that still contains electrons.
Valence electrons: The electrons of atoms that participate in the formation of chemical bonds.
Lewis symbols: Symbols of the elements with their number of valence electrons represented as dots
Electronegativity: The tendency of an atom or molecule to attract electrons and form bonds.
Covalent bond: A type of chemical bond where two atoms are connected to each other by the sharing of two or more electrons.
Cation: A positively charged ion.
Anion: A negatively charged ion.
Polyatomic ion: A charged species composed of two or more atoms covalently bonded, or of a metal complex that acts as a single unit in acid-base chemistry or in the formation of salts. Also known as a molecular ion.
Formal charge: The charge assigned to an atom in a molecule, assuming that electrons in a chemical bond are shared equally between atoms. This helps determine which of a few Lewis structures is most correct.
Resonance structure: A molecule or polyatomic ion that has multiple Lewis structures because bonding can be shown in multiple ways.
Coordinate covalent bond: A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from the same atom.
Lewis acid: Any substance, such as the H + ion, that can accept a pair of nonbonding electrons; an electron-pair acceptor.
Lewis base: Any substance, such as the OH- ion, that can donate a pair of nonbonding electrons; an electron-pair donor.
Lewis acid-base adduct: A compound that contains a coordinate covalent bond between the Lewis acid and the Lewis base.
Bonds and Interactions for the MCAT: Everything You Need to Know
Learn key MCAT concepts about bonds and interactions, plus practice questions and answers
(Note: This guide is part of our MCAT General Chemistry series.)
Table of Contents
Part 1: Introduction to bonds and interactions
Part 2: Interatomic forces
a) Ionic bonds
b) Covalent bonds
c) Coordinate covalent bonds
d) Single and double bonds
Part 3: Intermolecular forces
a) Hydrogen bonds
b) Dipole-dipole interactions
c) London dispersion forces
Part 4: High-yield terms
Part 5: Passage-based questions and answers
Part 6: Standalone questions and answers
Part 1: Introduction to bonds and interactions
What holds everything in the universe together? Why don’t atoms and molecules randomly split apart and float away? The answer lies in bonding and molecular interactions. Understanding how atoms and molecules interact with each other is crucial for much of the chemistry section of the MCAT. Atomic bonds and intermolecular attractions are the foundation for many additional concepts in organic chemistry and biochemistry.
Chemical bonds are chemical interactions that create an attractive force between atoms or molecules. There are a variety of bonds, or attractive forces, that exist. Some exist between individual atoms of a molecule, while others exist between molecules.
Throughout this guide, there will be several important terms marked in bold. Be sure to understand these terms well, as they will be high-yield on Test Day! At the end of this guide, there are also several MCAT-style questions you can use to test your knowledge.
Let’s get started!
Part 2: Interatomic forces
a) Ionic bonds
The simplest form of interatomic bond is known as an ionic bond. Ionic bonds are formed through the interaction of two atoms with vastly different electronegativities. Because both atoms have different electronegativities, one atom donates electrons to another. This results in the creation of a positively charged ion (cation) and a negatively charged ion (anion). The negatively and positively charged atoms attract, creating an ionic bond.
Because ionic bonds take advantage of different electronegativities, the constituent elements usually have quite different characteristics. In fact, ionic bonds are typically composed of metal and nonmetal. Sodium chloride (chemical formula: NaCl) is a molecule that is held together by an ionic bond. Sodium is a highly reactive metal, and chlorine is a highly reactive gas. Together, they form table salt, which we consume every day!

b) Covalent bonds
The second form of interatomic bond is the covalent bond. A covalent bond is usually formed between two atoms with similar electronegativities. Thus, covalent bonds are usually formed between nonmetals or metalloids.
Because the atoms have similar electronegativities, the atoms do not donate electrons to each other. Instead, they share electrons. This sharing between the two atoms can either be equal or unequal. Whether atoms in a covalent bond equally or unequally shared electrons depends on their difference in electronegativity. The greater the difference in electronegativity between two elements is, the more partial ionic character (e.g., the more similarity to an ionic bond) the bond between them has.
For instance, the water molecule (chemical formula: H2O) is held together by covalent bonds. The electronegativity of oxygen is greater than hydrogen. Thus, an oxygen atom will hold a greater attraction for electrons. As a result, the sharing of electrons between the oxygen atom and two hydrogen atoms is unequal. This results in a directionality, or a polarity, to the bond. Thus, water contains polar covalent bonds.
The polarity of a covalent bond can be described through a dipole moment. The dipole moment is a quantity that can be calculated using the following equation:
where p = dipole moment,
d = distance between the partial charges
This equation may look similar to another law: Coulomb’s law, which describes the attractive force generated between two charged particles. Here, the dipole moment describes an analogous quantity. A large dipole moment indicates a larger polarity, or difference in electron distribution, between partial charges.
In contrast to polar molecules, elemental oxygen (chemical formula: O2) is held together by a nonpolar covalent bond. Both oxygen atoms in the molecule have equal electronegativities, preventing one atom from pulling electrons closer to itself. As a result, the electrons are shared equally.
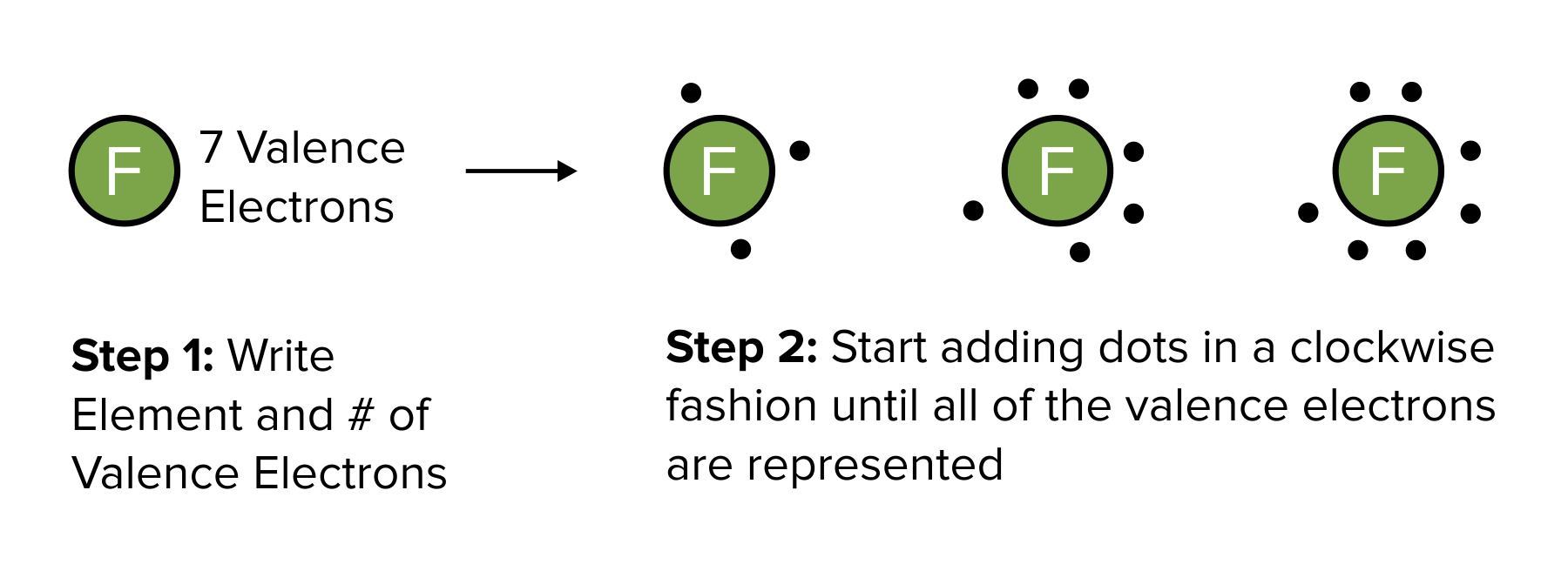
Lewis structures are a common method of representing covalent bonds. In Lewis dot structures, electrons in the valence orbitals are represented by a dot on each side of an atom’s elemental symbol. (To learn more about valence electrons, be sure to refer to our guide on atoms and periodic trends.)
To draw a Lewis dot structure, begin by writing the elemental symbol for the atom of interest. Then, calculate the number of valence electrons in the s and p orbitals. Once calculated, place one dot to the top of the elemental symbol. Continue placing dots in a clockwise manner around the symbol until the number of dots equals the number of valence electrons. This process is repeated for each element in a covalent bond.
After each atom participating in a covalent bond is illustrated, electrons participating in sigma bonds can be connected with a line.
Take a look at the Lewis structure for ozone below. Ozone (chemical formula: O3) has three unique oxygen atoms participating in single and double bonds. How do we know which oxygen atom is supposed to have the double bond?
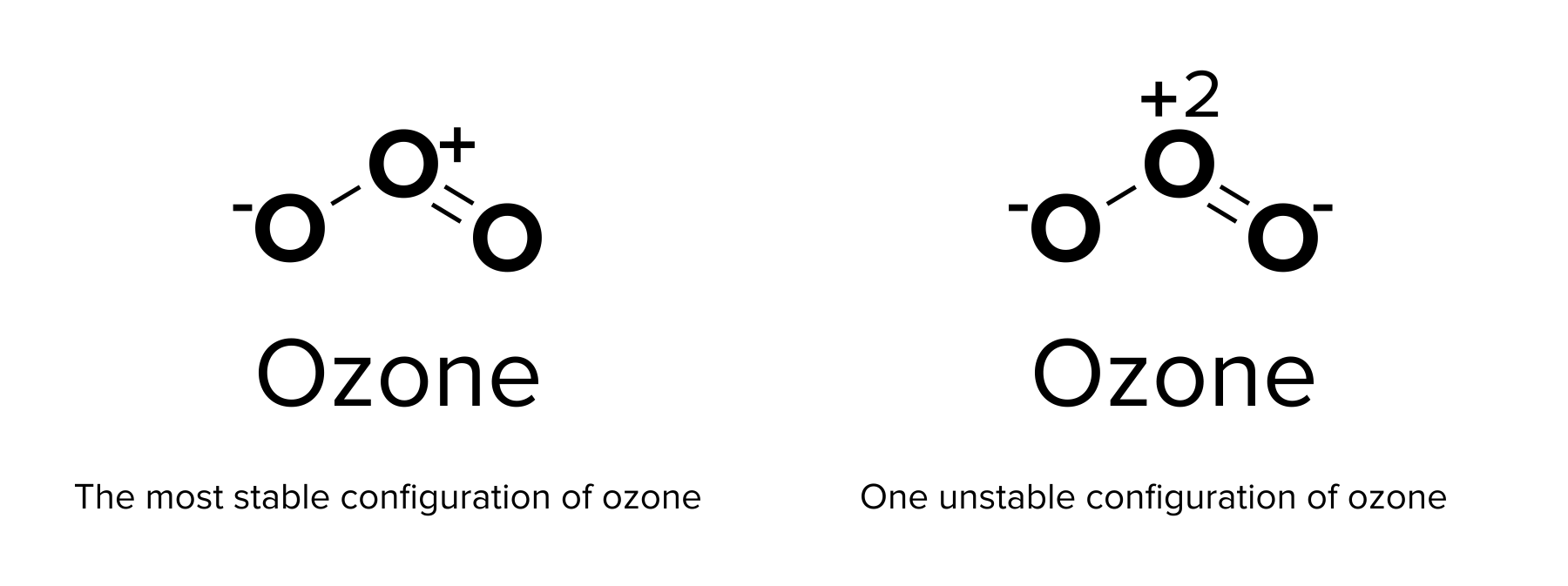
One advantage to using a Lewis dot diagram is that resonance structures can be shown. A resonance structure is an alternative model of covalent bonding. A molecule that displays resonance may have electrons in any of these resonance configurations at any given time. In general, the bonds within such a molecule are assumed to be a combination of all of these resonance structures. The electrons within the molecule are said to be delocalized and can be found with equal probability within any of these resonance structures. The resulting combined configuration may be shown with a dashed line.
The most stable resonance form is one that provides the lowest formal charge of the atoms. Formal charge refers to the amount of “functional” charge an atom would have, assuming all electrons are shared equally. Thus, it can be calculated using the following equation:
formal charge = # valence electrons – nonbonding electrons – bonding electrons ÷ 2
Recall our previous discussion of ozone. In the ozone molecule, each oxygen atom has a different formal charge. One atom has a charge of 0, while the other two have charges of -1 and +1. This is the relatively stable form of ozone, as the formal charges of each species are fairly low. In contrast, creating only single bonds in ozone would create formal charges of -1, -1, and +2, which are much higher formal charges. This resonance form is highly unlikely to occur!
Molecules and chemical compounds can often be drawn in a variety of ways. For more information on this, be sure to refer to our guide on isomers.
c) Coordinate covalent bonds
Recall that covalent bonds are simply bonds formed between atoms in which electrons are unevenly shared. A coordinate covalent bond, sometimes referred to as a dative bond, is a special subtype of covalent bond that often forms between transition metals. In this type of covalent bond, both electrons in the bond are donated by a single atom.
Due to their unique orbital structure, transition metals can easily accept electrons from other atoms. For this reason, transition metals can form two, three, or four coordinate covalent bonds with other atoms! Zinc, iron, and magnesium are examples of such metals that usually bond with enzymes inside our bodies. Since they are integrated into enzymes and are often critical for the enzyme’s function, these transition metals are referred to as cofactors. (For more information on enzymes and cofactors, be sure to refer to our guide on enzymes.)
d) Single and double bonds
In chemistry, the octet rule states that each atom is most stable when it contains a full valence shell of 8 electrons. This means that atoms with 1 electron in their valence shell are ready to give up their electrons while those with a nearly full shell aren’t. Thus, each atom in a molecule must be exposed to eight electrons: either through electron transfer (e.g. ionic bonding) or sharing (e.g., covalent bonding).









